Abstract
Background: T cell exhaustion is a homeostatic state that limits the immune system's ability to clear persistent antigen such as cancer or chronic infection. During the initial stages of antigen exposure, T cells have a robust response. Then proliferation and function dampen, and they settle into a state of exhaustion (TEX) marked by increased expression of inhibitory receptors. However, some cells in the exhaustion pathway retain effector function and gene signatures, a feature that is associated with expression of KLRs (killer lectin-like receptors) and effector genes, such as granzymes, with decreased expression of inhibitory receptors. Growing evidence in humans and mice suggests these cells are important cytotoxic effectors. Despite their potential benefit, the signaling molecules regulating the differentiation of this population are poorly understood. Our lab recently identified the pseudokinase Tribbles 1 (Trib1) as a novel regulator of T cell effector function during chronic infection (Rome et al, JEM 2020). In the current study, we use scRNA-seq, RNA velocity, flow cytometry, and TCR clonal analysis to demonstrate that deleting Trib1 shifts TEX towards KLR+CD8+ T cells (TKLR), away from a terminal TEX fate, and that loss of Trib1 augments anti-PDL1 blockade to improve viral control during chronic infection.
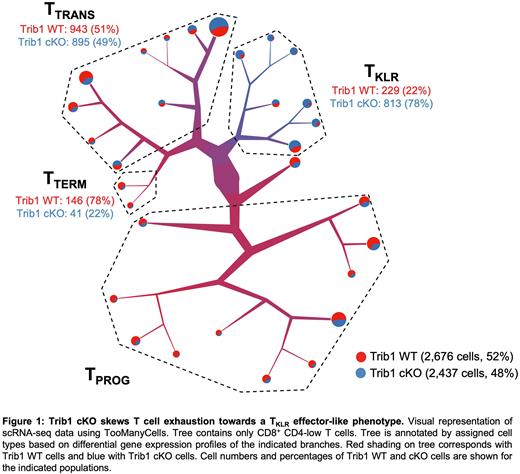
Methods and Results: We previously performed scRNA-seq on CD44+TCRβ+ T cells from WT (CD4-Cre+Trib1+/+) and Trib1 cKO (CD4-Cre+Trib1f/f) mice that were infected with LCMV clone 13 for 15 days. When CD4+ and CD8+ T cells were analyzed, we noted that a CD8+KLRG1+ cluster was uniquely enriched in Trib1 cKO cells compared to WT cells, and that this subset had a transcriptional profile strikingly similar to effector T cells from acute infection (Rome et al, JEM 2020). To better understand how Trib1 regulates CD8+ T cell biology during chronic infection, we specifically analyzed only CD8+ T cells from this data set and found that CD8+ Trib1 cKO cells are enriched for multiple KLR genes such as Klrk1, Klrg1, Klrc1, Klrd1, Klrb1c, Klre1, and KLR-associated genes such as Hcst (encoding Dap10), as well as Gzma and Cx3cr1 compared to WT cells, which express higher levels of exhaustion-associated genes such as Pdcd1, Tox, and Eomes. Total CD8+ cells were clustered using TooManyCells (Schwartz et al, Nat. Methods 2020), and based on gene expression profiles the tree was annotated to include progenitors (TPROG), transitional cells (TTRANS), terminally exhausted TTERM, and TKLR (Fig. 1). RNA velocity was used to predict the directional flow of cell differentiation states within the CD8+ cells in each cluster, and both WT and Trib1 cKO CD8+ cells progress from TPROG to TTRANS states, which then bifurcate into either TTERM or TKLR subsets. However, in Trib1 cKO mice, the propensity towards TKLR differentiation was stronger than in WT mice. scRNA-seq and flow cytometry confirmed that Trib1 cKO mice are enriched for the TKLR subset, while WT mice are enriched for TTERM cells, confirming this shift in cell fate decision (Fig. 1). Single cell TCR sequencing further revealed that Trib1 cKO CD8+ cells preferentially clonally expand at the TTRANS and TKLR stage, reinforcing that Trib1 deletion during T cell exhaustion redirects the fate of CD8+ cells towards an effector-like TKLR state. Finally, Trib1 deletion from T cells of mice treated with immune checkpoint inhibition using an anti-PDL1 antibody during chronic viral infection improves viral load (2.2 x 104 PFU/ml in Trib1 WT vs 9.1 x 103 PFU/ml in Trib1 cKO mice; p = 0.024) and this is associated with expanded TKLR subsets.
Conclusions: Trib1 deletion from mouse T cells during chronic infection intrinsically promotes a TKLR fate by increasing the expression of KLR-associated genes at all stages of exhaustion and by driving the clonal expansion and differentiation of TTRANS towards an effector-like TKLR identity. We demonstrate for the first time that deletion of a single signaling molecule can promote a TKLR fate while diverting the pathway of exhaustion towards a more effector-like state, and that this is accompanied by improved viral control. Additionally, Trib1 deletion improved anti-PDL1 treatment in chronically infected mice by enhancing the expansion of TKLR. Thus, manipulating Trib1, or it's signaling partners, could provide a novel means to improve T cell function during chronic antigen exposure, such as infection or cancer therapy.
Disclosures
Pear:Amgen: Current equity holder in private company; Johnson & Johnson: Current equity holder in private company; Pfizer: Current equity holder in private company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal